This week’s Diagnostics Evidence Accelerator meeting consisted of 2 presentation:
1. COVID-19 Test with Consumer-Collected Sample and Data Flow/Data Opportunities (Savitha Arokiamary, Amazon Dx, and Jim Daniel and Dr. Taha Kass-Hout, Amazon Web Services)
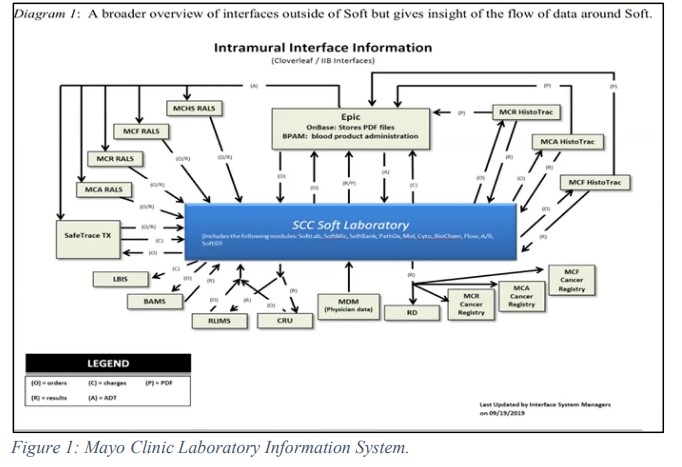
The public health reporting (PHR) team at Amazon Dx is responsible for reporting COVID-19 test results to all state public health leaders and reporting authorities. In order to achieve this, the team uses their internal reporting service engine where they established an electronic reporting process with all 50 states and DC. The electronic laboratory reporting (ELR) system is a transport mechanism used by the state agencies to share data, and in this case, the results of PCR- based COVID-19 testing. This reporting structure has three mechanism: Secure File Transfer Protocol (SFTP), Simple Object Access Protocol (SOAP) and Public Health Information Network Messaging System (PHINMS). Using the transport mechanisms, PHR shares the data via HL7, with the state reporting authorities. HL7 is a file type contains strings of data such as patients’ demographics and test information and collates into a single report. HL7 bridges the gap between the different health IT applications and allows the sharing of the data.
2. Funding Announcement (Reagan-Udall Foundation for the FDA)
As the pandemic has continued, the role of accurate and reliable SARS-CoV-2 diagnostics tests remain a critical component of the COVID-19 response. Some of the needs were assessed in Project One and we learned about important trends in sero-testing and performance of serological tests. Additionally, through Project One we learned a lot from our limitations such as the lack of interoperability to link manufacturer information with clinical and demographic data left us with a good deal of missing data on the actual test name and race. Therefore, the FDA Foundation is announcing a new program that can help us dig into the limitations from Project One.
The purpose of the funding is to
• Improve the use, interpretation, and performance of diagnostic tests as a means to:
• improve the medical products available to patients
• enable critical public health decision-making
• Expedite advances in knowledge regarding IVD performance, including strengths and limitations for use in different settings or subpopulations
• Enable a more rapid regulatory review process relative to current pre- and post-market data approaches • Increase knowledge of real-world performance of SARS-CoV-2 IVDs
• Inform a framework for IVD test developers to collect and analyze data for regulatory submission
• Can lead to regulatory submissions from SARS-CoV-2 IVD manufacturers for Emergency Use Authorization (EUA) or Full Market Approval (FMA)
Full January Lab Summary