Authors: Amy Cavet1; Claire Cravero, MPH2; Aaron Galaznik, MD3; Bray Patrick-Lake, MFS1; Aniketh Talwai3
Acknowledgments: Vera Mucaj, Bobby Samuels, Rahul Jain
1Evidation
2Datavant
3Medidata
Author Disclosures: Claire Cravero is an employee at and owns stock in Datavant. Bray Patrick-Lake owns stock in Evidation.
Background on Real-World Data
In response to the COVID-19 pandemic, use of real-world data (RWD) for research accelerated, supporting the global response to the pandemic via conduct of rigorous studies in the safety and effectiveness of diagnostics and of existing and newly approved therapies and vaccines in near real time. RWD include information recorded in the electronic health record (EHR), administrative claims, patient-reported outcomes (generally surveys about how a patient feels and functions), patient-generated health data (from apps, smartwatches, pedometers, etc.), product- and disease-specific registries, mortality data, and information about environmental factors and social determinants of health. Taken together, these RWD sources can enable a broad and specific picture of a patient’s journey and population health as a whole. The use of RWD has many advantages, most notably that data, typically collected as a part of routine health care, are available expeditiously and commonly for large populations. However, there are special considerations for the use of these data for the secondary purpose of research because they are created in individual health care systems for the primary purposes of patient care and billing.
Definitions from the Framework for FDA’s Real-World Evidence Program1
- Real-World Data (RWD): data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources.
- Real-World Evidence (RWE): clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD.
As part of the 21st Century Cures Act (Cures Act) of 2016, the Food and Drug Administration (FDA) created Framework for FDA’s Real-World Evidence Program to satisfy these provisions and bolster the use of RWD for approval of new indications for medical products and use in post-approval study requirements.1 More recently, the FDA published a series of draft guidance documents, including a guidance on “Real-World Data: Assessing Electronic Health Records and Medical Claims Data To Support Regulatory Decision-Making for Drug and Biological Products.”2 In the past two decades, large networks in the US, such as Sentinel3–5 and the Patient-Centered Outcomes Research Network (PCORnet),6–8 were launched to use administrative claims and electronic health record data for evaluation and research purposes. Globally, other countries have built similar data repositories, particularly countries with universal health care systems.
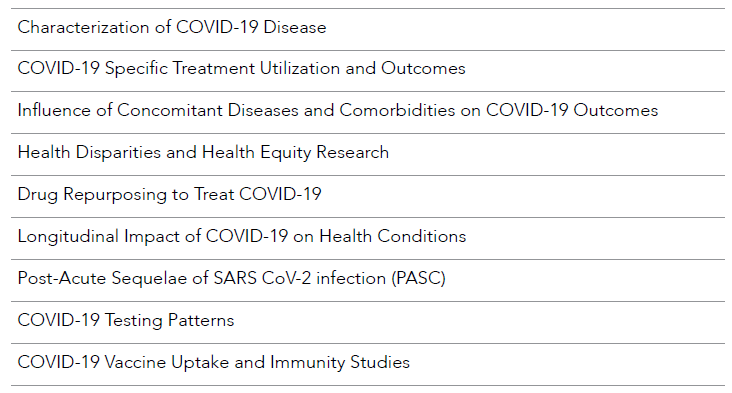
When the COVID-19 pandemic started, a foundation had been laid such that researchers could effectively launch studies to investigate COVID-19-related questions using RWD, including the burden of COVID-19 infection, the effectiveness of therapies (pharmacologic and non-pharmacologic treatments and vaccines), and the impact of programs and policies on disease outcomes.9 RWD are poised to address key themes, such as those listed in Table 1.1.
Table 1.1. Example Themes to Explore with RWD
Use of RWD may allow researchers to quickly generate clinical evidence while reducing the burden and cost of research conduct in some cases as compared to randomized controlled trials. Importantly, the RWD must be fit-for-purpose – that is, reliable, valid, and relevant. While not every research question can be addressed with RWD, the ability to generate real-world evidence (RWE) if adequate RWD are available depends further on the application of appropriate methodology based on epidemiologic principles to answer the research question.1
