Author: Iwagami M
What is it used for?
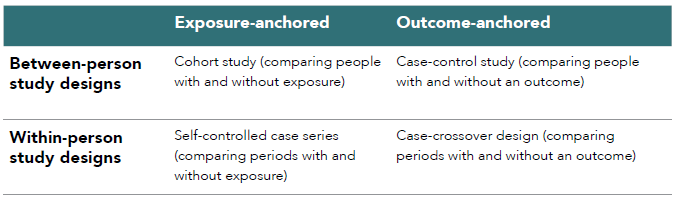
Within-person study designs, also known as self-controlled study designs, case-only designs, or Self-controlled Crossover Observational PharmacoEpidemiologic (SCOPE) studies, mainly include self-controlled case series (SCCS) and case-crossover (CCO).62 These designs compare different time windows (i.e., lengths of time) within the same person, instead of comparing different people in between-person study designs such as cohort and case-control studies. An SCCS compares the occurrence of an outcome (event) during periods with and without exposure in the same person, whereas a CCO compares periods with and without the outcome for exposure (see Figure 2.4). Table 2.2 contrasts the between-person and within-person study designs.
The within-person study designs are used for electronic health record (EHR) research, sometimes together with the between-person study designs for the same research question. In addition, when primary data collection is newly planned, the within-person study design can be efficient, because only data for people with the outcome of interest are needed. In addition, the within-person study designs are feasible if most people in the study population were already exposed (to a vaccine for children,63 for example), whereas a small number (proportion) of non-exposed people make the between-person study designs infeasible.
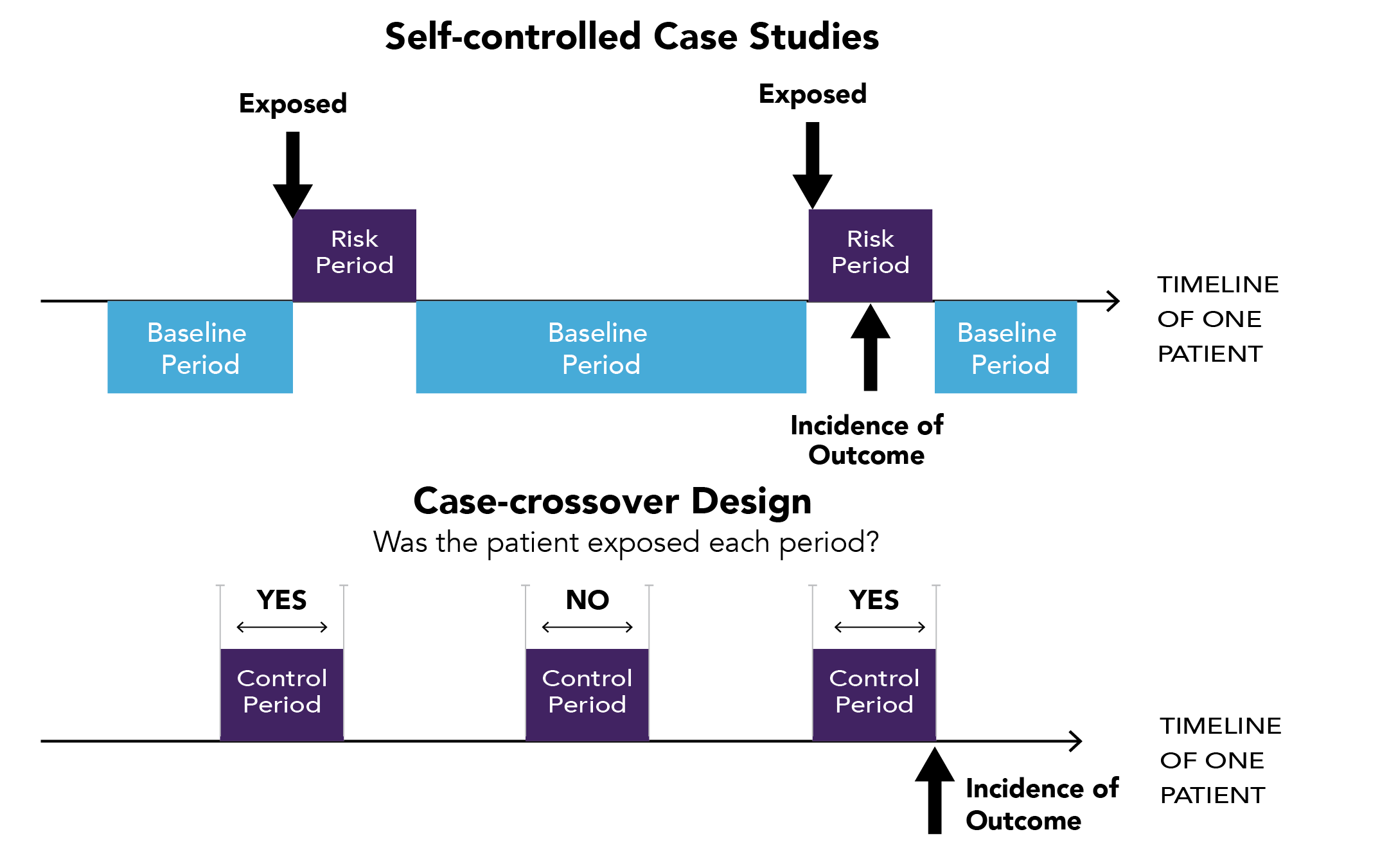
Figure 2.4. Graphical representation of self-controlled case series (SCCS) and case-crossover (CCO)
Table 2.2. Between-person and within-person study designs
What kinds of questions can be addressed?
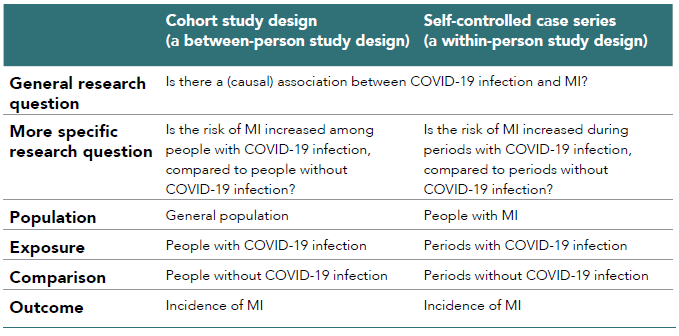
Between-person study designs and within-person study designs can be used for the same research question, applied somewhat differently.64 Table 2.3 shows the difference of population, intervention/exposure, comparison, and outcome [PI(E)CO] between a cohort study and SCCS for the research question on the association between COVID-19 infection and myocardial infarction (MI). The more specific research question that has been tailored to each study design is somewhat different. However, if there was a true causal association between COVID-19 and MI, both study designs should find a statistically significant association between them and reach the same conclusion (provided that they were well powered and appropriately conducted, minimizing biases inherent in each study design).
Table 2.3. Difference of PI(E)CO between a cohort study and self-controlled case series for an example of the association between COVID-19 and myocardial infarction (MI)64
What are the benefits and limitations of this design?
The main strength of within-person study designs is that they can minimize confounding factors that do not change over time (e.g., sex, genetics, habitual healthy or unhealthy behaviors). This is because the effects of these factors are cancelled out by design, even if they are unknown or unmeasured. Therefore, the within-person study designs can be used when there is concern about unmeasured or unknown confounding factors in between-person study designs. There are some examples in which an apparent association between an exposure and an outcome in a between-person study design was not confirmed by the within-person study design, possibly suggesting unmeasured confounding inherent in the between-person study design (or violation of the key assumptions that would allow use of the within-person study design, as shown below).65
However, within-person study designs cannot be used for all research questions. The SCCS and CCO designs require several assumptions specific to each study design. In the SCCS, (i) occurrence of an event should not (appreciably) affect subsequent exposures or opportunity for subsequent observation, which can happen when, for example, the outcome is fatal, (ii) the background risk for the event must be constant within intervals, and (iii) events must be independently recurrent or rare.66 In the CCO there should be no substantial change in exposure trends during the study period. Assumptions for both designs are best met when the exposure is transient (intermittent) and the outcome onset is abrupt (sudden), and there is no within-person confounding within the periods of observation.67 If these assumptions are violated, within-person study designs can result in biased estimates.
Judging whether (or not) these assumptions are met is case-by-case, usually based on biological and clinical knowledge, whereas some statistical tests have been proposed to test (part of) the assumptions.67 An article from Petersen et al. shows an example of how to assess SCCS assumptions, with some solutions for if the assumption is violated, for the association between COVID-19 vaccination and thrombocytopenia and thromboembolism events, which was indeed examined within SCCS design using UK electronic health records.66,68,69
