Author: Lerro C
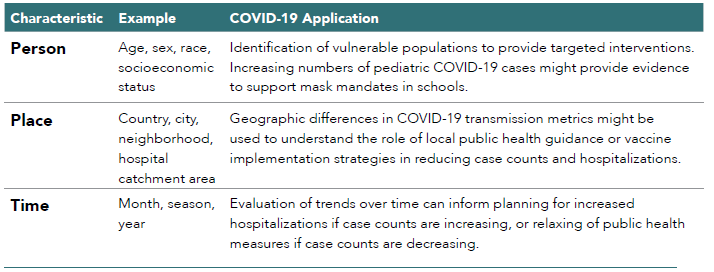
Descriptive epidemiology involves analysis of disease or exposure patterns in the population, focusing on characteristics related to person, place, and time (see Table 2.4).70 Descriptive studies are broadly used for both scientific and administrative public health purposes.
For scientific purposes, descriptive studies can inform our understanding of disease etiology. We can make inferences about the underlying causes of disease by monitoring how disease incidence and prevalence change over time; differ with respect to age, sex, and race; and vary geographically. These studies cannot answer causal questions. However, information gleaned from descriptive studies might be used to generate hypotheses for high-quality rigorously controlled observational studies or trials.
Descriptive studies are used for administrative purposes to establish public health priorities, allocate funding, and evaluate the effectiveness of public health interventions. A health department monitoring uptake of COVID-19 vaccination might observe a sharp increase following implementation of a vaccine lottery, most pronounced in persons age 20-29. These descriptive results might indicate that this strategy is effective in motivating younger adults to get vaccinated.
In the context of the COVID-19 pandemic, descriptive data have become ubiquitous as a means of informing the public about patterns of testing, treatment, and disease (see Table 2.4 for examples of person, place, and time in descriptive studies of COVID-19). These descriptive data allow individuals and governments to take steps to protect personal and public health, respectively. Worldwide, many localities and federal agencies regularly publish information on case counts, vaccination rates, testing, hospitalizations, and deaths and use descriptive data to monitor the medical product supply chain to anticipate potential drug shortages.
Table 2.4. Person, Place, and Time in Descriptive Studies
What kinds of questions can be assessed?
Descriptive studies are best suited to assessing patterns (person, time, place) of disease and exposure incidence and prevalence. In the context of pharmacoepidemiology, the exposure of interest is often a therapeutic or vaccine. Descriptive studies of drug utilization can demonstrate inappropriate use of therapeutics and underutilization of necessary therapeutics, including the impact of drug shortages on patterns of utilization and vice versa. A study conducted across Denmark, Finland, Iceland, Norway, and Sweden described public health measures as well as patterns of clinical management and outcomes of patients with severe COVID-19 in the ICU during the first wave of the COVID-19 pandemic.71 Authors found substantial variability in the critical care response across these countries, including in the use of invasive mechanical ventilation and use of certain treatments (e.g., IL-6 antagonists, hydroxychloroquine, and corticosteroids). A strength of this study is that it uses high-quality data to evaluate demographic factors, clinical characteristics, and patterns of ICU care across several countries, allowing authors to hypothesize potential reasons for variation in ICU mortality rates that could be addressed further using an inferential study design. This study also highlighted limitations of the RWD that were used; for example, some variables were missing for certain countries including use of specific types of therapeutics and certain comorbidities such as obesity.
A descriptive analysis in the National COVID Cohort Collaborative evaluated inpatient use of hydroxychloroquine, remdesivir, and dexamethasone among 137,870 US adults hospitalized with COVID-19.72 The authors were interested in evaluating both prevalence of use and trends in use over time. Hydroxychloroquine and remdesivir received emergency use authorization (EUA) from the Food and Drug Administration (FDA) for treatment of COVID-19 during 2020 (hydroxychloroquine EUA was later rescinded) and remdesivir and dexamethasone were included in the National Institutes of Health treatment guidelines for some adults hospitalized for COVID-19. The authors found that 6.3%, 21.2%, and 39.1% of the 137,870 patients were treated with hydroxychloroquine, remdesivir, and dexamethasone, respectively. Trends in use over time indicated that hydroxychloroquine use increased following the EUA and started declining after the EUA was revoked. The authors hypothesized that lower use of remdesivir compared to dexamethasone might be due to reported remdesivir shortages during the study time period. Following release of results of the RECOVERY trial, which demonstrated clinical benefit of dexamethasone among patients with mechanical ventilation or oxygen, 78-84% of patients who had invasive mechanical ventilation were treated with dexamethasone or other corticosteroids. The findings suggest that dexamethasone might be underutilized among patients who are mechanically ventilated, and that wide variation in patterns of dexamethasone and remdesivir use across health centers indicates differences in patient case mix, drug access, treatment protocols, and quality of care. The lower-than-expected use of dexamethasone and variation across health centers might have been a consequence of drug shortages, indicating that hospitals restricted use to patients who were critically ill.
In a multinational study conducted across 11 data sources in 4 countries (the US, Spain, China, and South Korea), authors described the use of repurposed and adjuvant drugs in patients admitted to the hospital with COVID-19.73 Commonly used repurposed drugs included hydroxychloroquine, azithromycin, combined lopinavir and ritonavir, and umifenovir, and the prevalence of use across data sources and countries varied widely. Though trends in use of repurposed drugs varied widely over time and often increased or decreased following regulatory guidance or highly publicized trial results, trends across data sources and even countries were generally similar. Authors reported large variation in use of adjunctive drugs across data sources, with the 5 most used treatments being enoxaparin, fluoroquinolones, ceftriaxone, vitamin D, and corticosteroids. This study fills an important knowledge gap by describing the drugs most used in this patient population and comparing use across different institutions worldwide. Inferential studies might be used to quantify the risk and benefit of these treatments in the management of patients with COVID-19.
Descriptive studies can also inform our understanding of disease etiology and might be used to identify vulnerable individuals who are at greater risk of disease compared to the general population. For example, early descriptive studies in the COVID-19 pandemic noted that rates of COVID-19 infection and COVID-19 mortality were higher among individuals with pre-existing hypertension.74 Based on this descriptive evidence and biologic plausibility, several observational studies evaluated this association with rigorous control for potential confounders, yet found no clear relationship between hypertension and severe COVID-19 independent of age, sex, and other risk factors.
What are the benefits and limitations of this design?
An important strength of descriptive studies that has not previously been highlighted is that they can often be done at low cost with existing data. Many descriptive studies can repurpose existing surveillance data to answer their research questions. For example, studies have used vital records and census data to estimate excess deaths attributed to the COVID-19 pandemic in many countries, including the US, Italy, Norway and Sweden, England and Wales, Guatemala, and Korea. Other descriptive studies, particularly those seeking to evaluate patterns of drug utilization, might instead use large existing administrative databases.75
Descriptive studies are not suited to answering causal research questions. This study design does not involve comparative analysis or rigorous consideration of concerns known to impact interpretation of observational studies that aim to make causal inferences including confounding, measurement bias, and selection bias. A descriptive analysis of monoclonal antibody use in a large claims data source highlights both the capabilities of descriptive designs as well as the limitations.76 Of the 211 million patients in the data source, 69,377 received monoclonal antibody treatments from January 2020 through April 2021. Bamlanivimab accounted for 85% of administered treatments, though trends indicated that combination bamlanivimab and etesevimab use was increasing. The study used outpatient data only, and without data from the inpatient setting, results underestimate overall monoclonal antibody use. The authors found that patients who received monoclonal antibodies were less likely to have Medicaid insurance compared to patients in the database overall, indicating possible barriers to care for these patients. Among patients receiving monoclonal antibodies, missing data was systematically lower for certain variables (e.g., race/ethnicity and household income) compared to patients in the database overall, which might impact the inferences made regarding these patient characteristics.
Descriptive studies are unique in that the purpose is often to generate research questions for inferential analyses as opposed to providing definitive answers. In addition, they might be able to provide important data to inform public health and clinical decision-making even in settings where there would be significant challenges to conducting a well-designed inferential study. When used correctly, descriptive studies can be a powerful tool to inform our understanding of disease etiology, inform public health, and monitor drug uptake and utilization.
